My name is Luis Escudero. For over 10 years, I have been fortunate to work as a physiotherapist in the field of football, and for the last five years, in professional football. During this time, I have gained hands-on experience in managing and rehabilitating musculoskeletal and sports injuries, particularly anterior cruciate ligament (ACL) injuries. This experience has allowed me to understand the limitations of traditional methodologies, delve into the personal management of elite athletes, and recognize the need to integrate new technologies to optimize outcomes while engaging athletes in an effective yet enjoyable manner. For over three years, I have been a member of “Team ACL,” a leading group dedicated to research and dissemination on this pathology.
ACL Rehabilitation in 2025
Navigation:
- How do I find a good ACL physical therapist?
- How do I find a good ACL doctor?
- Zamst
- Why are so many female footballers suffering ACL injuries?
- Aspetar Clinical Practice Guideline on rehabilitation ACLR
- Melbourne ACL Rehabilitation Guide
- HOW an athlete returns is more critical than WHEN
- Anterior Cruciate Ligament (ACL) Injury Hidden in the Complex Sensorimotor System
- ACL Reference Values
- Psychological readiness
- Virtual Reality and Artificial Intelligence: Transforming Injury and ACL Rehabilitation
- ACL rehab staying motivated
- The Team ACL effect
- Keiser
- Game Ready Pro 2.1.
- Compex
- ACL reconstruction rehabilitation: decades of change
- Reference values for quadriceps and hamstrings strength & double- and single-leg jump tests
- Return to running too quickly after ACL surgery
- Blood Flow Restriction Training in ACL Rehab
- Interview TeamACL professional Luis Escudero
- Influence of female sex and graft choice on the incidence of cyclops lesions after ACL reconstruction
- 10 Mistakes why ACL rehab regularly fails
- Isokinetic testing after ACL rehabilitation in football players
- Return to sports after an ACL reconstruction in 2024
- Latest content
ACL Rehabilitation in 2025:
“Cross Bracing (CBP) and Aspetar Guide as symbols of a measurable new paradigm”
The anterior cruciate ligament (ACL) is one of the primary passive and dynamic stabilizers of the knee joint, limiting anterior tibial translation relative to the femur and providing rotational stability (Duthon et al., 2006). ACL injuries are particularly prevalent in sports involving abrupt directional changes, rapid decelerations, and pivoting maneuvers, accounting for up to 64% of all knee injuries in elite athletes (Grassi et al., 2020). Ligament rupture significantly disrupts joint biomechanics, and inadequate management may result in persistent instability, secondary meniscal injuries, and accelerated progression toward post-traumatic osteoarthritis (Filbay, Grindem, & Roos, 2022).
Therapeutic approaches to ACL injuries encompass both surgical and conservative strategies. In physically active individuals, ligament reconstruction remains the standard of care (Ardern, Webster, Taylor, & Feller, 2018). However, the clinical success of such intervention depends not only on the surgical procedure itself but also on the implementation of a rehabilitation protocol based on functional criteria, which ensures the progressive recovery of strength, joint stability, and neuromuscular control required for a safe return to sport (Grindem, Snyder-Mackler, Moksnes, Engebretsen, & Risberg, 2016).
In this context, the conceptual evolution of clinical guidelines has led to a gradual departure from time-based rehabilitation models, favoring strategies guided by objective parameters. The Aspetar Clinical Practice Guideline is among the most widely recognized international references, prioritizing functional recovery over the time elapsed since surgical intervention (Kotsifaki, Korakakis, King, Barbosa, & Maree, 2023). In contrast, guidelines such as OPTIKNEE 2022 adopt a more conservative approach focused on long-term prevention of post-traumatic osteoarthritis, although they do not entirely discard the use of functional progression criteria (Ibrahim, Mayr, Ziegler, & Suedkamp, 2022).
This article aims to critically examine ACL rehabilitation from an updated perspective, with a focus on the model proposed by the Aspetar guideline. Each phase of the rehabilitation process will be addressed through a practical and evidence-based lens, incorporating progression criteria, common errors, and clinical recommendations. Additionally, the Cross Bracing Protocol (CBP)—an emerging conservative model that has garnered considerable attention in recent scientific discussions—will be introduced. The CBP advocates for ligament self-repair guided by structured imaging and functional progression (Filbay et al., 2023).
Historical Evolution of ACL Reconstruction Techniques
Surgical intervention on the anterior cruciate ligament (ACL) has undergone substantial evolution over the past six decades, driven by the continuous pursuit of enhanced functional stability, reduced failure rates, and more efficient biological integration of the graft. In the 1960s and 1970s, primary repairs through direct suture techniques demonstrated clinically unacceptable failure rates (Feagin & Curl, 1976), leading to a progressive shift toward more stable reconstructive approaches.
In the 1980s, the autologous bone–patellar tendon–bone (BPTB) graft emerged as the surgical gold standard, particularly among high-demand athletes (Clancy et al., 1982). During the 1990s, hamstring grafts—semitendinosus and gracilis—gained popularity, aiming to reduce donor site morbidity (Fu et al., 1999). In the early 2000s, the use of allografts became widespread due to their surgical convenience and shorter recovery time; however, subsequent studies reported significantly higher failure rates in young and athletic populations (Poehling et al., 2005).
The 2010s marked the rise of a more integrative biomechanical approach, with the introduction of adjunct techniques such as lateral extra-articular tenodesis and anterolateral reinforcement, which improved rotational control and reduced the risk of graft re-rupture in high-risk patients (Getgood et al., 2019). More recently, since 2023, advancements in biological surgery—including the application of growth factors and stem cell therapies—have opened new avenues in clinical research aimed at optimizing tendon-to-bone integration and mitigating postoperative joint degeneration (Seitz et al., 2023).
Rather than reflecting surgical trends alone, this evolution illustrates a transition toward more personalized and multifactorial therapeutic models that integrate biomechanical, biological, and functional elements. These approaches aim to maximize clinical outcomes, minimize complications, and facilitate a high-quality return to sport.
ACL reconstruction has thus undergone multiple innovations throughout the years

This table clearly outlines the theoretical progression of ACL reconstruction techniques over the decades. However, ACL rehabilitation is currently undergoing a silent revolution. For years, reconstructive surgery followed by function-based rehabilitation—such as that advocated by the Aspetar Clinical Practice Guideline—has been the undisputed standard of care. Yet in recent years, and even in the past several months, a new approach has begun to gain traction among clinicians seeking more conservative and biologically driven alternatives: the Cross Bracing Protocol (CBP).
This is a strategy that does not begin with the scalpel, but rather with clinical reasoning, diagnostic imaging, and the body’s inherent capacity for self-repair. In subsequent sections, we will describe the CBP in detail, step by step—not as a return to outdated methods, but as a rediscovery of the body’s regenerative potential when given time and structure. The CBP is not a universal alternative, but it is a viable option for selected cases—and that distinction alone reshapes the conversation. Selection, monitoring, and measurable progression are the true keys to the CBP’s success.
2025 — Healing of acute anterior cruciate ligament rupture on MRI and outcomes following non-surgical management with the Cross Bracing Protocol Filbay, S. R., et al. (2023)
ACL surgery has evolved toward more personalized techniques, incorporating anterolateral reinforcement and biological augmentation strategies to optimize clinical outcomes.
In the field of orthopedic surgery, it is not uncommon to observe the cyclical resurgence of certain techniques, driven by new research findings, technological advancements, or shifts in clinical trends. A paradigmatic example is the lateral extra-articular tenodesis, commonly known as the Lemaire technique, which is often used as an adjunct to anterior cruciate ligament (ACL) reconstruction. This technique, originally developed by Marcel Lemaire in the 1960s, aimed to stabilize the knee by using a fascia lata graft to reinforce anterolateral rotational stability. The graft is passed beneath the lateral collateral ligament and fixed to the femur (Muñoz-Garrote et al., 2020).
1967 — Old ruptures of the anterior cruciate ligament of the knee: Functional treatment
with extra-articular plasty Lemaire, M. (1967)
For years, the Lemaire technique was a widely used option to address rotational instability of the knee. However, with the advent of intra-articular ACL reconstruction techniques and the growing emphasis on anatomical approaches, its use gradually declined. Nevertheless, a resurgence in the application of this technique is currently being observed. This renewed interest is partly due to studies demonstrating that isolated ACL reconstruction may be insufficient to control rotational laxity in certain patient populations, particularly in young, hyperlax individuals or those engaged in pivoting sports (Zaffagnini et al., 2017).
The reintroduction of the Lemaire technique in contemporary practice is supported by its ability to enhance rotational stability and reduce failure rates in ACL reconstructions—especially in revision procedures or in patients with marked rotational instability. Recent studies suggest that combining intra-articular ACL reconstruction with lateral extra-articular tenodesis can improve functional outcomes and decrease the incidence of graft re-ruptures (Sonnery-Cottet et al., 2017).
It is important to reflect on the cyclical nature of such “surgical trends” and to recognize that the reappearance of techniques like the Lemaire procedure is not merely a matter of fashion, but rather a response to the ongoing quest for better clinical outcomes based on current evidence. The key lies in the meticulous assessment of each patient, considering individual risk factors and selecting the most appropriate surgical tools for each case. As such, the Lemaire technique—far from being an outdated method—represents a valuable addition to the therapeutic arsenal in ACL surgery, and its application should be guided by sound, patient-specific clinical criteria.
As previously discussed, ACL reconstruction techniques have undergone significant advancements in recent decades, allowing for more effective restoration of joint stability. There are currently three primary approaches to ACL surgical management:
A. ACL Reconstruction Using Autografts
Definition: This approach involves using the patient’s own tissue to replace the ruptured ligament.
Autograft options:
- Patellar tendon (Bone–Patellar Tendon–Bone, BPTB) → Considered the gold standard for high-performance athletes (Barber-Westin & Noyes, 2020).
- Hamstring tendons (Semitendinosus and Gracilis) → A less invasive technique associated with reduced postoperative pain (Samuelsson et al., 2019).
- Quadriceps tendon, with or without a bone block → Frequently used in revision procedures or in patients with compromised tissue quality (Mohtadi et al., 2021).
Advantages
+ Lower risk of graft rejection
+ Superior biological and mechanical integration
Disadvantages
– Potential donor site weakness, particularly with hamstring and quadriceps grafts
B. ACL Reconstruction Using Allografts (Tissue Bank Grafts)
Definition: This technique involves the use of cadaveric tendons preserved in tissue banks.
Allograft options:
- Posterior tibialis tendon
- Achilles tendon
- Peroneus longus tendon
Advantages
+ Avoids donor site morbidity
+ Enables less invasive procedures and shorter surgical time (Seitz et al., 2019)
Disadvantages
– Higher risk of graft failure, particularly in young, active patients (Crawford et
al., 2020)
– Potential for immune reaction and transmission of infections
C. Extra-articular Plasty and Reinforcement Techniques
Definition: Techniques such as anterolateral tenodesis or anterolateral ligament (ALL) reconstruction aimed at enhancing rotational stability.
Clinical use:
- Indicated for patients with high-grade rotational instability
- Often combined with intra-articular ACL reconstruction to reduce re-rupture rates in young athletes (Getgood et al., 2022)
Advantages
+ Improved control of rotational pivot, reducing residual laxity
Disadvantages
– More complex techniques with increased surgical time
Criteria for Surgical Technique Selection
The choice of surgical approach depends on biomechanical, clinical, and patient-specific factors (Grindem et al., 2021).

Conclusion:
- Elite athletes → BPTB graft or combined with ALL reinforcement
- Low-demand patients → Hamstring autograft or allograft
- Cases with rotational instability → Combined anterolateral plasty
THE ASPETAR CLINICAL PRACTICE GUIDELINE
The Aspetar Clinical Practice Guideline provides a systematic framework for rehabilitation following anterior cruciate ligament (ACL) reconstruction, grounded in functional and measurable criteria. This paradigm replaces traditional time-based models, emphasizing the achievement of specific clinical and functional benchmarks before progressing through each rehabilitation phase. The following outlines the phases proposed in the guideline, along with their respective objectives and criteria for progression:
Phase 1: Early Recovery (0–2 weeks)
Clinical objectives:
- Pain and inflammation control
- Restoration of full knee extension
- Neuromuscular activation of the quadriceps
Progression criteria:
- Full extension without pain (0°)
- Minimal or absent joint effusion (grade 0–1, stroke test)
- Active quadriceps contraction (SLR without extension lag)
- Tolerance to partial weight-bearing without symptom exacerbation
Phase 2: Restoration of Mobility and Initial Strength (2–6 weeks)
Clinical objectives:
- Recovery of full range of motion
- Normalization of gait pattern
- Initial activation and symmetry of extensor and flexor muscle groups
Progression criteria:
- Knee flexion ≥ 120°
- Uncompensated gait under full weight-bearing
- Quadriceps strength ≥ 60% compared to the contralateral limb
- Ability to perform 10 pain-free mini-squats (30°–40°)
Phase 3: Strength Development and Neuromuscular Control (6–12 weeks)
Clinical objectives:
- Increased functional load through closed kinetic chain exercises
- Improvement in strength symmetry and postural control
Progression criteria:
- Knee flexion ≥ 135° without restrictions
- Quadriceps strength ≥ 70% (measured via dynamometry or manual testing)
- Single-leg balance ≥ 30 seconds without compensations
- Ability to perform 3 controlled single-leg squats at 45°
Phase 4: Reintroduction of Low-Impact Activity and Basic Agility (3–6 months)
Clinical objectives:
- Gradual return to running and initial plyometric exercises
- Consolidation of dynamic stability during basic sport-specific tasks
Progression criteria:
- Single-leg hop test ≥ 85% of the contralateral side
- Triple hop test ≥ 85% of the contralateral side
- Execution of 10 drop jumps with controlled landing mechanics
- Illinois Agility Test completed without visible compensations
Phase 5: Controlled Return to Sport (6–9 months)
Clinical objectives:
- Progressive simulation of sport-specific movements
- Improvement in reactivity and control during multidirectional activities
Progression criteria:
- T-test for change of direction without compensations
- Hop test and triple crossover hop ≥ 90% of the contralateral limb
- Ability to decelerate and pivot without pain or instability
- ACL-RSI score ≥ 90% (psychological readiness for return to sport)
Phase 6: Full Return to Competition (9–12 months)
Clinical objectives:
- Restoration of full athletic functionality
- Prevention of re-injury through complete functional symmetry
Medical clearance criteria:
- Symmetrical performance across all functional tests
- Limb Symmetry Index (LSI) > 95%
- Ability to complete training at pre-injury volume and intensity
- Passing of sport-specific testing protocols
This progression model not only ensures a safer recovery but also facilitates individualized, data-driven clinical decision-making, minimizing the risk of reinjury or chronicity in the rehabilitation process.

Common Errors in ACL Rehabilitation and Evidence-Based Solutions
In ACL rehabilitation, success is not only determined by executing the right interventions but also by avoiding critical missteps. Progression is not solely dictated by prescribed exercises, but by the clinician’s ability to recognize and prevent errors that may compromise graft integrity, disrupt biomechanics, or increase the risk of reinjury. A premature jog, a jump performed with asymmetric force, or advancing phases without adequate neuromuscular control can delay recovery and jeopardize long-term functional outcomes. Rehabilitation is not merely about following a program—it is about making clinical decisions informed by data and the patient’s demonstrated capacities, not just the time elapsed since surgery.
The consequences of bypassing objective progression criteria are often not immediately evident but may manifest in the patient’s long-term return-to-sport performance or reinjury risk. Therefore, knowing when not to progress is just as important as knowing when to advance.
The following table summarizes common rehabilitation errors across phases, their potential consequences, and evidence-based corrective strategies (adapted from Aspetar Clinical Guidelines):
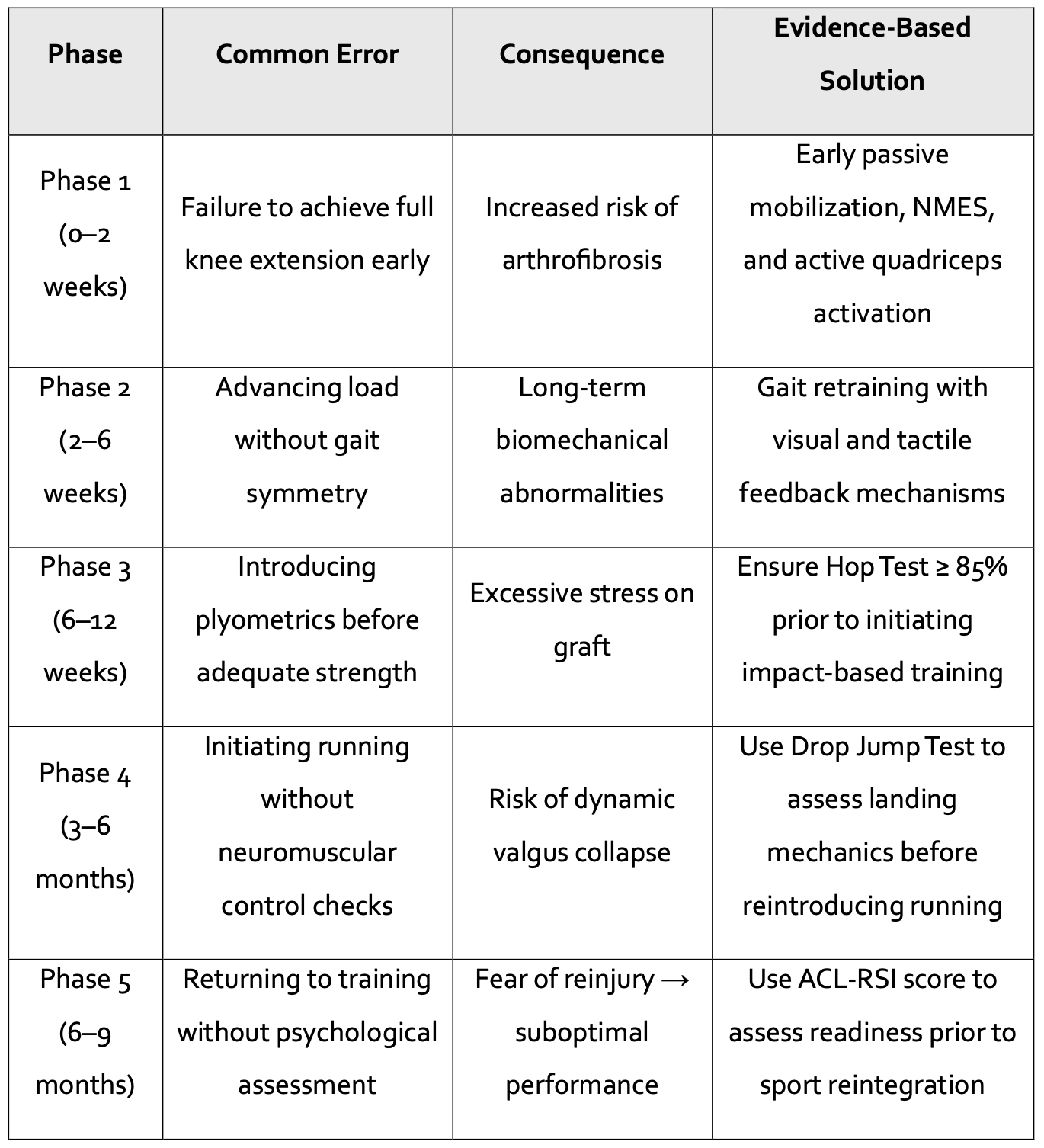
Table: What, When, and How to Apply Advanced Therapies in ACL Rehabilitation
Aspetar recommends the use of several advanced therapeutic modalities; however, the optimal timing and application are not always clearly defined. This table summarizes their practical implementation.

The systematic integration of these preventive measures not only reduces complication rates but also reinforces the role of the physiotherapist as a key clinical decision-maker throughout the entire rehabilitation process.
Final Reflection: A Paradigm Shift Beyond Time-Based Models
The consolidation of protocols such as the Aspetar Clinical Practice Guideline and the Cross Bracing Protocol (CBP) does not merely represent a methodological evolution in anterior cruciate ligament (ACL) rehabilitation—it constitutes a true inflection point in contemporary clinical practice. Although they originate from different therapeutic perspectives—Aspetar following surgical reconstruction and CBP as a conservative alternative—both approaches converge on a fundamental principle: the replacement of standardized chronological progression with individualized, criterion-based rehabilitation.
This emerging paradigm stands in marked contrast to traditional models, epitomized by protocols such as Shelbourne and Nitz (1990), which advocated for accelerated return to sport at six months without objective functional validation, or conventional postoperative guidelines for bone–patellar tendon–bone (BPTB) grafts, which organize recovery around predetermined weekly phases. Even widely adopted institutional frameworks—such as those of the American Academy of Orthopaedic Surgeons (AAOS)—continue to apply rigid time-based criteria, often neglecting the clinical variability inherent to each patient.
Current evidence suggests that time-driven rehabilitation is not only insufficient but potentially harmful. Advancing phases without concrete functional benchmarks may expose patients to avoidable risks, including graft re-rupture, arthrofibrosis, or long-term performance deficits. In contrast, both Aspetar and CBP integrate objective tools such as limb symmetry indices (LSI), hop tests, psychological readiness scales (e.g., ACL-RSI), and, in the case of CBP, advanced imaging parameters, to ensure safe progression tailored to the individual’s functional profile.
This is not merely a technical distinction—it represents a profound shift in the philosophy of rehabilitation: moving from rigid, prescriptive practices toward functional, personalized, and evidence-based medicine. Since ACL injuries do not impact all patients equally, it is illogical to assume that all should progress at the same rate.
Therefore, the discussion is no longer about which guideline to choose, but about definitively moving away from inflexible calendar-based models. The true innovation lies in embracing rehabilitation as an ongoing process of assessment, measurement, and clinical decision-making—not merely the passage of time.
Nevertheless, it is important to highlight that despite its growing popularity and promising early clinical outcomes, the CBP remains under scientific scrutiny. A significant proportion of existing studies has been produced by research teams with ties to commercial entities involved in the protocol’s development, introducing potential conflicts of interest that warrant cautious interpretation. Rather than diminishing its value, this context underscores the critical need for further independent investigation to determine CBP’s true potential in redefining conservative management of ACL injuries. The fundamental question that remains unanswered is whether surgical intervention is truly necessary in specific patient profiles.
Ultimately, the present and future of ACL rehabilitation must be founded on personalization and clinical precision. The use of objective data is not a trend—it is a requirement aligned with the highest standards of care. In this regard, Aspetar and CBP are not opposing paths, but rather two converging expressions of the same paradigm shift: one in which clinical criteria prevail over the calendar.
Comparative Table: Criterion-Based Protocols vs. Time-Based Protocols in ACL Rehabilitation

Author: Luis Escudero Soria
Member of: Team ACL
Specialization: Physiotherapy and Sports Rehabilitation

References
- Ardern, C. L., Webster, K. E., Taylor, N. F., & Feller, J. A. (2018). Return to sport following anterior cruciate ligament reconstruction surgery: A systematic review and meta-analysis of the state of play. British Journal of Sports Medicine, 52(22), 1286–1296. https://doi.org/10.1136/bjsports-2016-097403
- Barber-Westin, S. D., & Noyes, F. R. (2020). Factors used to determine return to unrestricted sports activities after ACL reconstruction. Arthroscopy, 36(8), 1932–1951. https://doi.org/10.1016/j.arthro.2020.03.013
- Crawford, S. N., Waterman, B. R., & Lubowitz, J. H. (2020). Long-term failure of anterior cruciate ligament reconstruction. The Journal of Bone and Joint Surgery, 102(1), 35–44. https://doi.org/10.2106/JBJS.19.01083
- Duthon, V. B., Barea, C., Abrassart, S., Fasel, J. H., Fritschy, D., & Menetrey, J. (2006). Anatomy of the anterior cruciate ligament. Knee Surgery, Sports Traumatology, Arthroscopy, 14(3), 204–213. https://doi.org/10.1007/s00167-005-0679-9
- Filbay, S. R., Grindem, H., & Roos, E. M. (2022). Osteoarthritis risk following anterior cruciate ligament injury and reconstruction: What to tell patients and why. British Journal of Sports Medicine, 56(10), 558–559. https://doi.org/10.1136/bjsports-2021-104704
- Getgood, A., Bryant, D., Litchfield, R., Heard, M., & Peterson, D. (2022). Lateral extra-articular tenodesis reduces failure of ACL reconstruction. The American Journal of Sports Medicine, 50(4), 869–878. https://doi.org/10.1177/03635465221081035
- Grassi, A., Macchiarola, L., Filippini, M., Lucidi, G. A., D’Hooghe, P., & Zaffagnini, S. (2020). Epidemiology of anterior cruciate ligament injury in Italian first division soccer players. Journal of Sports Medicine and Physical Fitness, 60(2), 321–328. https://doi.org/10.23736/S0022-4707.19.10140-3
- Grindem, H., Snyder-Mackler, L., Moksnes, H., Engebretsen, L., & Risberg, M. A. (2016). Simple decision rules can reduce unnecessary anterior cruciate ligament reconstructions: The Delaware-Oslo ACL cohort study. British Journal of Sports Medicine, 50(10), 1216–1223. https://doi.org/10.1136/bjsports-2016-096646
- Ibrahim, M. M., Mayr, H. O., Ziegler, P., & Suedkamp, N. P. (2022). OPTIKNEE guidelines: A novel approach for long-term knee health following ACL injury. Clinical Journal of Sport Medicine, 32(4), 451–458. https://doi.org/10.1097/JSM.0000000000000974
- Kotsifaki, R., Korakakis, V., King, E., Barbosa, O., & Maree, D. (2023). Aspetar clinical practice guideline on rehabilitation after anterior cruciate ligament reconstruction. British Journal of Sports Medicine, 57(7), 500–514. https://doi.org/10.1136/bjsports-2022-106158
- Samuelsson, K., Andersson, D., & Karlsson, J. (2019). Autograft versus allograft in ACL reconstruction. Knee Surgery, Sports Traumatology, Arthroscopy, 27(7), 2387–2399. https://doi.org/10.1007/s00167-019-05397-5
